Okay, the title sounds a bit extreme at first, but let me explain.
Yes, atoms have nuclei (and electrons), and yes we have some models for them, but all of the primary models are wrong (though slightly insightful in their inadequacies). Recall what you probably have seen since childhood:
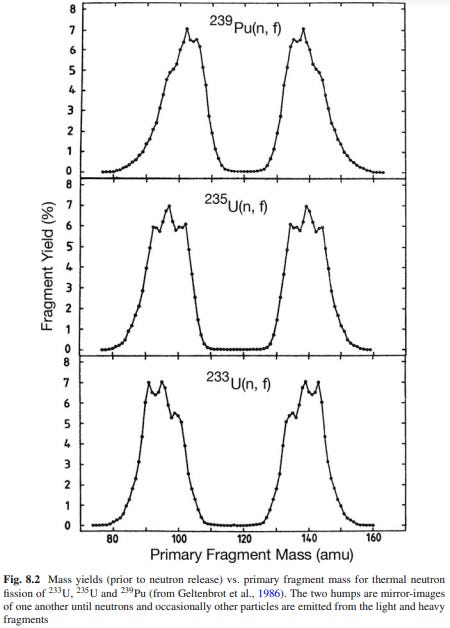
The vague ‘bunch of little balls’ model from the 1940s is good enough for bombs and reactors, and we haven’t really progressed successfully from that with anything predictive. All models are always wrong to some degree but we’ve really stalled on this one. The cold fusion/LENR situation in the 1990s prompted some review of nuclear models, and Norman Cook’s summary of nuclear models is stellar. It should be required reading for anyone interested and I will routinely reference (poach) details (mostly data) from it. He points out a number of important things, including the fact that asymmetric fission is completely unexplainable using existing models despite signs of obvious structuring. Kind of a big deal that we don’t know how fission works in detail when our reactors exploit it, right? (keen observers will note that cathode rays work whether you know they’re electrons or not, but JJ Thompson did eventually sort us out). The weirdness comes from the difficulty of explaining asymmetry from a shell/lattice/drop model, and the odd patterning of the clusters formed:
The error bars are supposedly within the data points on these curves, in which case we have a serious mystery that suggest some internal structure we don’t comprehend. The little clump of spheres model shows its inadequacy here - how would it split through the interior?
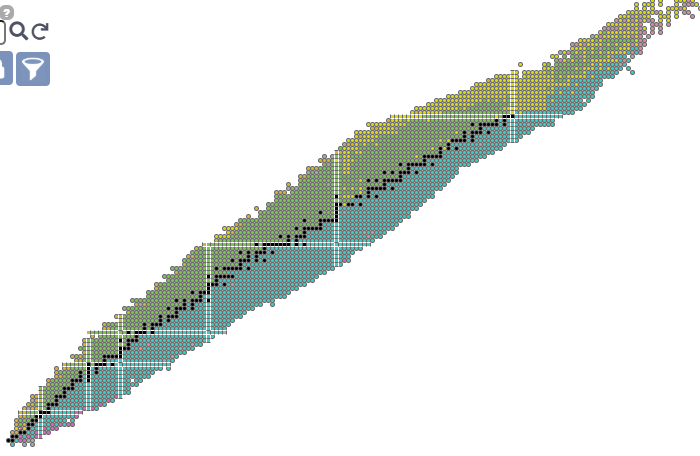
We can’t explain the Segre chart, with any degree of detail (This is actually my not-so-secret motivation behind my own shenanigans). It’s a big old pile of experimental data that keeps improving and it’s a damn puzzle.
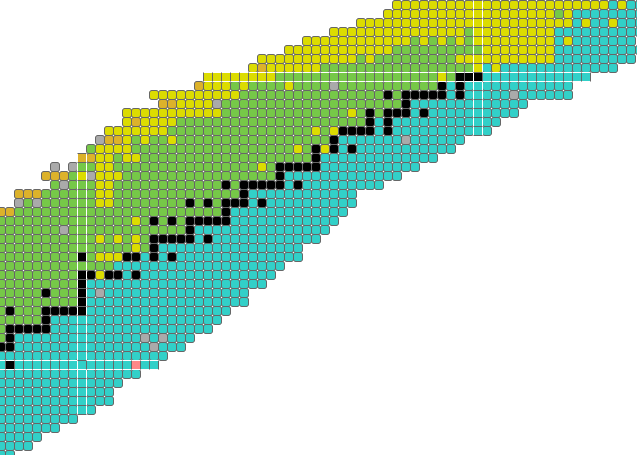
This image is stolen from the IAEA’s isotope explorer linked above. Stable isotopes are black, and form an OBVIOUS staircase structure, with additional stable points having a structured (but unspecified) relationship to the ‘staircase proper’. Isotopes which decay through alpha-emission are yellow, and these constitute a interesting subset of long lived isotopes in the unstable regions beyond Lead and Bismuth which continue the staircase. (Be8 is perhaps the prototype for alpha decay, but it is anomalously short lived.) Green isotopes decay by electron capture or positron emission, while blue isotopes undergo beta decay - both trending toward the staircase of stability. This decay-mode plot shows some interesting things. Proton and neutron emission occurs on the extreme edges, where the most ephemeral and least possibly useful isotopes briefly vibrate before rapidly transitioning back toward the more practical regions we still don’t understand.
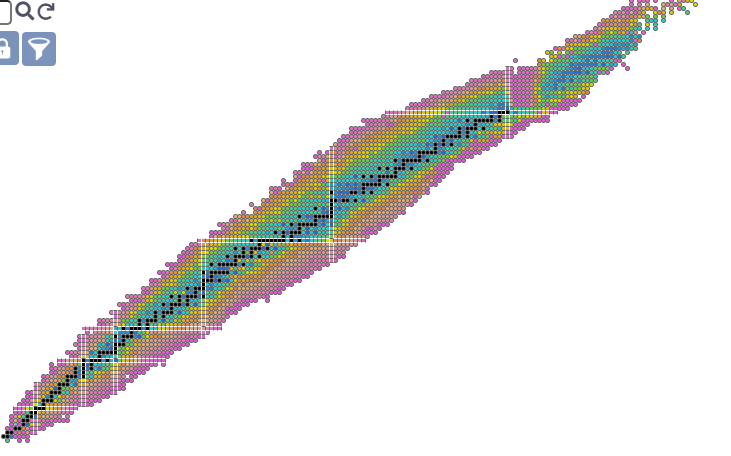
How don’t we understand it? Well let’s look at patterns in half-lives:
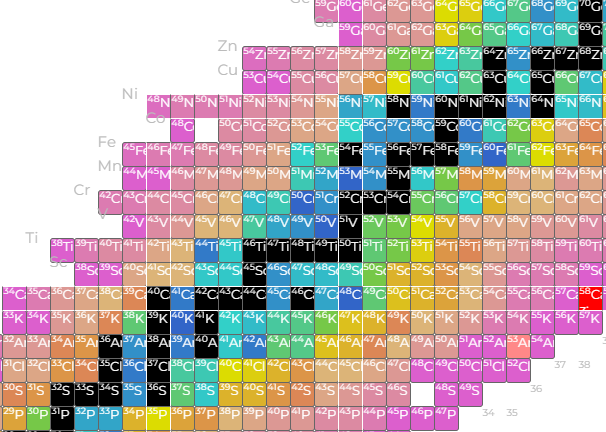
Stable (eternal unless modified?) isotopes remain black, but now the unstable ones have a color corresponding to their half life. The white bars are ‘magic number’ related (a cope if ever there was one). Less commented on is the observation of ‘cursed’ numbers, for which nothing stable exists. Technetium and Promethium are the primary cursed elements, along with neutron numbers 19, 21, 35, 39, 61, 71, 89, 115, and 123.
One might include 84 neutrons as well (kinda weird see below), but Nd144 has a *reported* half life of on the order of petayears, and there are other long lived isotopes at n=84. 109 neutrons appear similarly ‘almost stableish’, as W183 is more long lived than that. It should be remembered that all elements larger than Lead are unstable but sufficiently long lived to be mined, processed, etc. The heavy transuranics don’t seem to quite care whether or not they’re on the staircase. You can still make out the staircase structure, but even the unstable stuff has a pretty long lifetime.
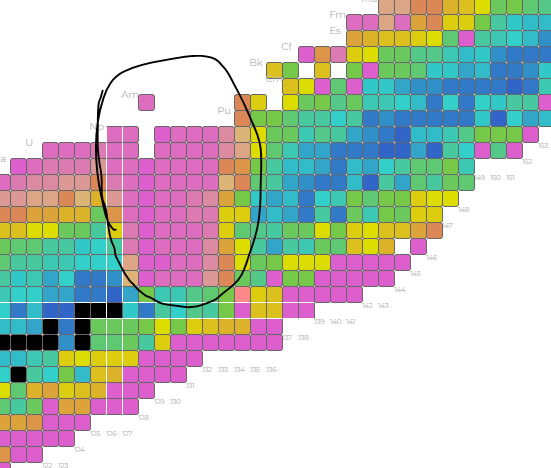
Soft stability boundaries
While the stability staircase structure shows an isotope-specific condition for stability (a ‘hard line’), there are also some softer boundaries which exist with no clear explanation.
What’s up with this gap? Sure, stability ends, but then some ‘structured metastability’ or something gives longer lifetimes to the staircase again. Why would that disappear? Everything gets bigger and bigger and then it’s just unstable…..but then if you ADD MORE TO IT and then it becomes stable again? This is obviously weird, and perhaps the source of the ‘island of stability’ idea that seems still elusive. That said, it’s not clear that we ever bothered to explain what this gap is about.
Forget about THAT weird stability wall for a second, lets go back to the decay mode graph:
At 84 neutrons (all of a sudden?) we get an even cursed neutron number, and suddenly alpha decay becomes much more common. This is where I start to wonder how good the data is toward the edges - alpha decay on the staircase makes sense. On the fringes *of instability* I’m not sure it’s worth focusing on too much, but if the signal is real, it deserves some consideration about what the hell is going on.
Here’s another soft boundary:
Apologies to the colorblind, but notice the transition from blueish (kinda stablish) radioisotopes to orange-to-pink (very unstable) radioisotopes. It’s more clear on the proton-rich side of the staircase (above), but someone somewhat similar seems to occur on the neutron rich side too (below), with some additional complexity. That’s kinda weird - what’s that about? It doesn’t really kick off until after Argon (abundance be damned, Ar38 looks ‘centered’, and is surrounded by two cursed neutron numbers so it’s interesting)
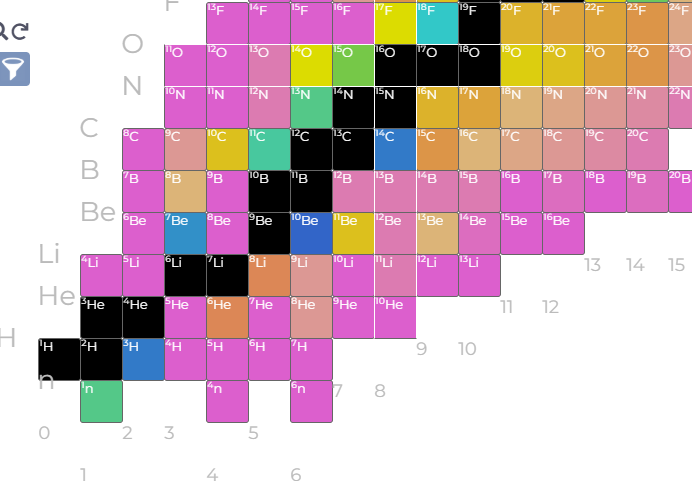
Even the simple stuff is a bit mysterious:
So, anything pink is so unstable we can’t even measure a half life for it - an energy associated with the decay is reported instead. Be8 is a bit odd but we often wave our hands about how super stable He4 is, and so Be8 just commits atomic seppuku to become two alpha particles (Is this the smallest fission, or is just earliest alpha emission? Perhaps this is not important but it stands out a bit). Be9 being stable is rationalized as the result of an extra neutron ‘gluing’ the two alpha particles together - fair play. So why is Be7 as stable as it is?
While we’re snooping around on this, how well do we understand beta decay again? Scott Locklin’s excellent post on the subject notes that periodicity in half lives seems to be a thing. Seems important.
It is perhaps worth noting at this point that there has been a more recent nuclear model proposed by Edo Kaal et al, called the Structure Atom Model (SAM). Edo was kind enough to send me a copy to read after I asked some interested questions, and i’m about half way through. I may review the concept in detail to point out the good/bad/ugly of the idea, but nobody wants to hear someone call their baby ugly and so some care is needed in this regard. It is important to state that Edo deserves substantial credit for summoning a substantial amount of effort and courage, and the SAM is perhaps getting at something which the other models have not. (Correct in principle, not specific detail)
More to come on the topic, but this hopefully this has pointed out a few odd things (drawn from reliable experimental observations) in need of better understanding. While there are over a dozen international organizations dedicated to nuclear science - it’s not clear that any of this bothers them much. Perhaps they’re mostly cutouts for nuclear reactor IP holders, but they seem somewhat incurious in any case.